
YWT provides a broad range of solutions for treating water and waste water. YWT’s main clients are industrial plants, particularly in the pharmaceutical sector, power, food, chemical and steel industries.
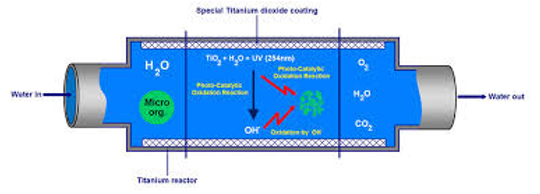
In chemistry, photo-catalysis is the acceleration of a photoreaction in the presence of a catalyst. In catalysed photolysis, light is absorbed by an adsorbed substrate.
In photo generated catalysis, the photocatalytic activity (PCA) depends on the ability of the catalyst to create electron–hole pairs, which generate free radicals (e.g. hydroxyl radicals: •OH) able to undergo secondary reactions. Its practical application was made possible by the discovery of water electrolysis by means of titanium dioxide.
The commercially used process is called the advanced oxidation process (AOP). There are several ways the AOP can be carried out; these may (but do not necessarily) involve TiO2 or even the use of UV light.
Generally, the defining factor is the production and use of the hydroxyl radical.

YWT provides a broad range of solutions for treating water and waste water. YWT’s main clients are industrial plants, particularly in the pharmaceutical sector, power, food, chemical and steel industries.